 |

Otolith Mark FAQ
What are Otoliths?
Parts of an Otolith
What is Thermal Marking?
Example of a Thermal Marked Otolith
Temperature Cycles Used to Thermal Mark
How can you find a Thermal Mark?
Measuring a Thermal Marked Otolith
Aging a Ground Otolith
Other methods of marking otoliths
Glossary of Terms
What Are Otoliths?
Otoliths or "ear bones" consist of three pairs of small carbonate bodies that are found in the head of teleost (bony) fish. Otoliths are primarily associated with balance, orientation and sound detection, and function similarly to incus, malleus and stapes in the inner ear of mammals. They occur in pairs, and each pair differs in location, function, size, shape, and structure. The three pairs of otoliths are called the lapilli, asterisci, and sagittae.
In Pacific salmon, the asteriscus and lapillus are usually quite small, only a millimeter in size, but the sagittae are much larger (~5 mm). Because the sagittae is so much larger than the other otoliths, they are easily recovered and are the most frequently studied. Sagittal otoliths are often referred to simply as "the otolith," although this term more correctly applies to all three structures.
The otolith is a crystal; consequently, it grows by the precipitation of ions on its exposed surfaces. During this process, protein and calcium carbonate are laid down on the surface of the otolith, although the relative amounts vary with time and season. Thin sections of an otolith reveal a detailed microstructure consisting of bands of opaque and translucent material, like the rings on a tree trunk. Fisheries biologists have discovered that they can assess a fish's life history by looking at changes in these patterns. An otolith's ring structure can provide information about an individual's age, growth rate, and environment. In some cases, these patterns are a natural record; in other cases they are induced by man.
Because otoliths provide useful information on age, growth rate, life history, recruitment, and taxonomy, they are widely used in fisheries management. Fisheries biologists like to think of otoliths as information storage units; a sort of CD-ROM in which the life and times of the fish are recorded. If we learn the code, we can learn about that fish.
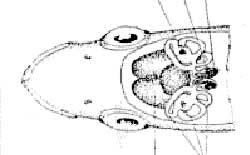 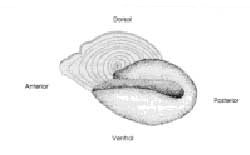
Parts of an Otolith (sockeye salmon)
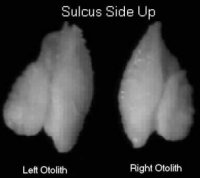
| This is an example of a sockeye otolith with the sulcus side up. This is the position in which the left an otolith is glued to a glass slide so that it can be ground down to the primordia and examined under a microscope to view the thermal mark. The second otolith can be used for aging. It is also useful if the first otolith gets damaged in preparation. |
 |
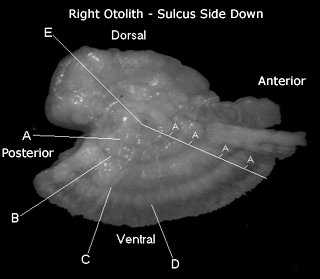 |
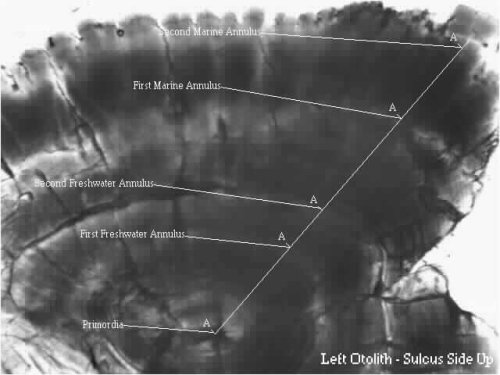
Here is an image of a right sockeye salmon sagitta that illustrates how a salmon is aged. Because sockeye salmon spend time in both freshwater and marine environments, their otoliths exhibit both freshwater and marine growth, as shown here. This fish is a 2.2 age fish, which indicates that it spent two years in freshwater then two years in saltwater before returning to its natal stream to spawn. To determine the total age of the fish, an additional year must be added to account for it's intragravel period. Consequently, an otolith aged as a 2.2 would have come from a 5-year old sockeye. The anterior portion of the otolith is the smaller, pointed end. The primordia is located in the center of the otolith.
A - First Freshwater Annulus
B - Second Freshwater Annulus
C - First Marine Annulus
D - Second Marine Annulus
E - Primordia
|
What is Thermal Marking?
An otolith is thermally marked by creating abrupt changes in water temperature during incubation. This induces a "dark ring" in the microstructure of a fish's otolith. Usually these rings are created by a rapid temperature decline of at least 3° Celsius followed by a cold interval of 24 to 48 hours. This disrupts normal otolith growth, and when the otolith is viewed under a microscope using transmitted light, a "dark ring" is visible. This ring contrasts sharply with the adjacent, narrow "white ring," which results from the relatively warm portion of the thermal cycle.
By planning a sequence of temperature changes, a hatchery is able to produce a pattern of dark rings in the otoliths of all fish exposed to those temperature changes.
Example of a Thermal Marked Otolith
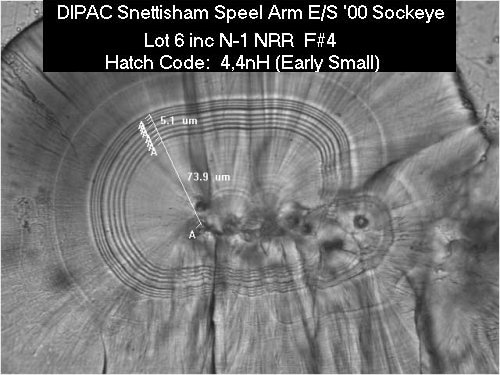
This is a photograph of a thin section of a sockeye salmon sagitta recovered from a 9-month old fry. This otolith was thermally marked at DIPAC's Snettisham Hatchery in Southeast Alaska. Using standard hatch code notation, this thermal mark is a 4,4nH pattern, which means that there is a band of 4 rings near the center of the otolith,which is followed by a space, then a band of 4 narrowly spaced rings (denoted by an "n" in the hatch code). The "H" indicates the position of the hatching event.
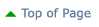
Temperature Cycles Used to Thermal Mark
This is a thermal marked sagitta from a sockeye salmon fry prior to its release in a lake. This fish was reared as part of Alaska Department of Fish and Game's International US/Canada Enhancement project.
The identifying thermal mark can be observed as a band of 7 dark rings. The temperature cycle that created this mark consisted of two days of warm temperatures followed by two days of cold temperatures.
To recover a thermal mark, the sagittal otoliths (the largest of the 3 pairs) are removed. The left sagittae is glued to a glass slide with thermoplastic cement and then polished on a grinder until the center of the otolith (e.g. primordia) is reached. Once polished, it is examined under a transmitted light microscope for the presence of a thermal mark.
Different numbers of rings are organized by groups or "bands" to create different patterns. These may by used to determine the hatchery from which the fish originated, as well as the year in which the fish was cultured.
How can you find a Thermal Mark?
To find a thermal mark on an otolith, fish must be sampled, then their sagittae otoliths (the largest of the 3 pairs) are removed.
The left sagittae otolith is glued to a glass slide with thermoplastic cement then ground on a grinder.
When the center of the otolith is reached, it is examined under a microscope for the presence of a thermal mark.
Different numbers of rings organized in "bands" create different patterns. These may indicate the hatchery from which the fish originated.
Measuring a Thermal Marked Otolith
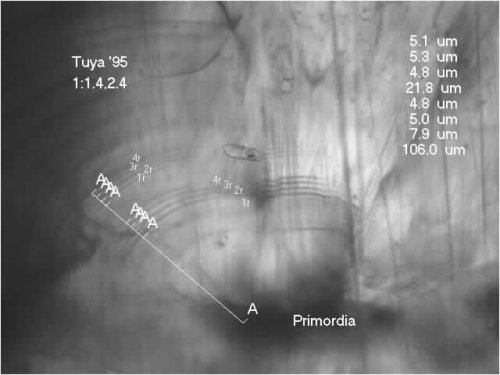
To quantitatively describe a thermal mark, we measure from the center of a primordia out to the mark located at the posterior of the otolith. Because salmonid otoliths have several primordia, we select the one closest to the posterior edge of the sagitta. The thermal mark encircles all primordia, but for consistency we try to measure to where the mark is located on the posterior of the otolith.
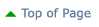
Aging a Ground Otolith (sockeye salmon)
This photograph shows a sagittal otolith from a sockeye salmon that was 2.2 years of age (i.e. two years of freshwater growth and two years of marine growth). This otolith was polished on a grinder down to the primordia so that light could pass through it and allow the ring structure to be observed. Each annual growth zone consists of two bands: a wide ring of dark material (spring and summer growth) and a narrow ring of relatively clear material (autumn and winter growth). The clear ring is often referred to as the annulus. Freshwater annuli tend to be closer together relative to marine annuli because growth is much greater while the fish is at sea.
Other methods of marking otoliths
Dry Marking
The dry method of otolith marking is based on periodic changes of the water level during incubation of the eggs. During one marking cycle, the eggs are kept in a dry, but humid, environment for 24 hours, and the eggs are subsequently washed with water during the next 24 hours. This cycle produces one dark and one light ring. To retain a humid atmosphere and prevent abrupt changes in temperatures, the incubators are covered with a plastic film or some type of heat-insulating material. Otoliths may be marked using this method during the eyed-egg stage until hatching. Unlike thermal marking, post-hatch marks cannot be applied.
In general, the method is simple, convenient and requires no special equipment. The resulting otolith marks are visually identical to the rings created by the thermal marking process.
Fluorescent Substances
Thermal marking of otoliths appears to be a benign and universal method of marking embryonic salmonids. It does, however, require that fish be cultured for an extended period. In some instances, it is desirable to quickly mark wild fish in situations where thermal marking is not practical.
Trefethen and Novotny (1963) recommended that stable isotopes be used to create recognizable marks. Since then, investigators have exposed salmonids to a variety of bone-seeking fluorescent substances with varying degrees of success. The widespread use of these methods will depend on the cost efficiency and acceptance by regulatory agencies.
These chemicals include:
Alizarin
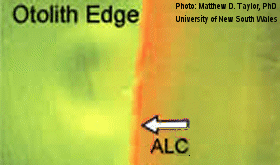
| The Japanese have used alizarin complexone (ALC) to mass mark chum salmon. Eggs were immersed for 24 hours in a solution of ALC (200 mg/l) with 1 N KOH before dilution. This fluorescent mark is only visible when the otolith is viewed under a UV light microscope. |
 |
Calcein
 |
Calcein is another fluorescent chemical marker that has recently been used to mass-mark fish. When fish are exposed to calcein by immersion or ingestion, it is absorbed into the body and incorporated into calcified structures, such as otoliths, fin rays, vertebrae, and scales. Once incorporated, it is stable and concentrations reportedly do not diminish with age. Calcein fluoresces under ultraviolet light and can easily be detected using fluorescence microscopy. |
Oxytetracycline
|
Oxytetracycline is a frequently used antibacterial agent that also binds to calcified tissues to create a yellow fluorescent mark when viewed under ultraviolet light. It can be introduced to the fish by injection or ingestion, and can be applied to any age group. Although several researchers have used OCT to validate age, some studies have reported that these marks may not permanent. Mark retention is believed to decrease with increasing age, and the application process does not always mark 100% of the target fish (Reinert et al. 1998).
|
 |
|
Reinert, T.R., J. Wallin, M.C. Griffin, M.J Conroy, and M.J. Van Den Avyle. 1998. Long-term retention and detection of oxytetracycline marks applied to hatchery-reared larval striped bass, Morone saxatilis. Can. J. Fish. Aquat. Sci. 55: 539-543.
|
Strontium
 |
Salmonids can be successfully mass-marked using strontium chloride at any life history stage. Thermal marks, in contrast, can only be applied during a 2 to 4 week period after the eyes form in the embryos. One drawback of the strontium mark, however, is that they cannot be viewed using a traditional microscope. They are only detectable using an electron microscope equipped with an electron backscatter detector. |

|
 |